A+
A-
Peninsula Medical gets China NMPA Class III for MFUS device
Writer: Lian Jiaqi | Editor: Zhang Chanwen | From: Shenzhen Daily | Updated: 2025-11-12
On Nov. 3, Shenzhen-based Peninsula Medical officially received a Class III medical device registration certificate from the National Medical Products Administration (NMPA) for its independently developed MFUS Ultrasonic System.
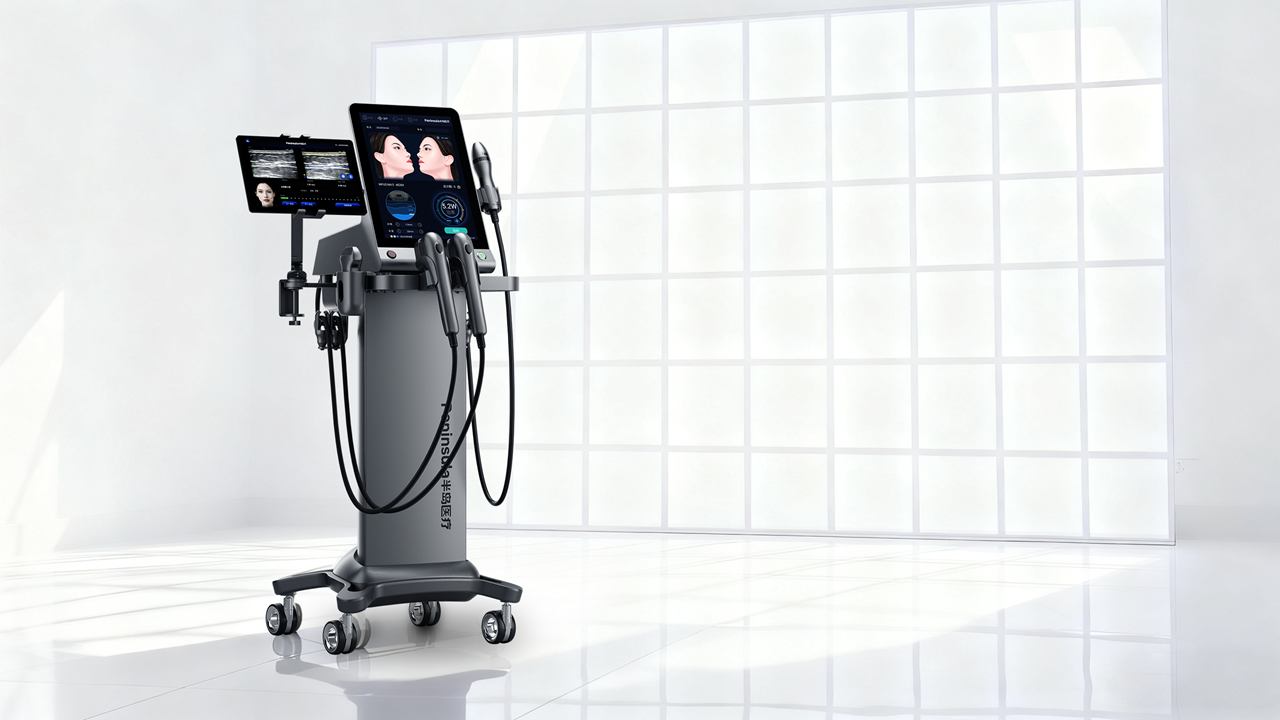
Peninsula Medical's independently developed MFUS Ultrasonic System. File photo
The clearance authorizes use of the device in medical institutions to deliver focused ultrasound thermal energy to subcutaneous tissues to improve skin laxity in the mid- and lower face, submental area and neck.
The approval represents a notable regulatory milestone for focused-ultrasound technologies in medical aesthetics in China.