Regulator approves use of homegrown oxygen device
The first domestically made extracorporeal membrane oxygenation (ECMO) was approved for use by China’s top drug regulator.
ECMO is a complicated life support device often used as a last-ditch treatment method for patients exhibiting critical organ failure.
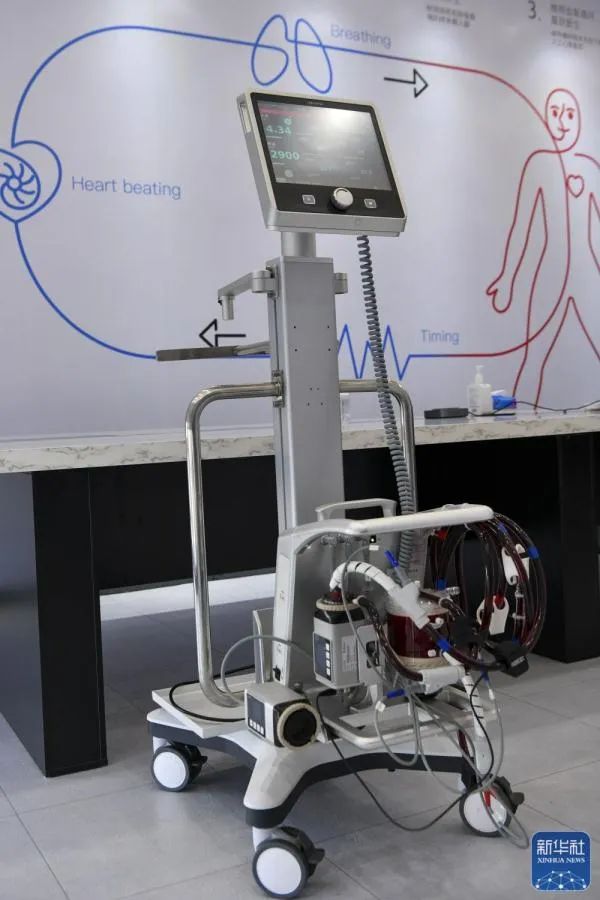
The Shenzhen-made extracorporeal membrane oxygenation (ECMO) recently approved for use. Xinhua
The equipment, developed by Shenzhen Chinabridge Medical, is on par with its international counterparts, according to the National Medical Products Administration.
At a ceremony marking the issuance of the approval od the first domestically made ECMO on Friday, Xin Guobin, deputy minister of the Ministry of Industry and Information Technology, said the application of the homegrown ECMO is a milestone for the development of high-end medical equipment in China.
The issuance of the approval marked the country’s major breakthrough in ECMO in terms of its design, research, manufacturing and core spare parts.
The products will play an important role in meeting clinical demands and guaranteeing treatment for critical COVID-19 patients.
The ECMO’s research and manufacturing is headed by the National High-performance Medical Apparatus and Instruments Innovation Center and participated by Chinabridge Medical, Shenzhen Institute of Advanced Technology of Chinese Academy of Sciences and Mindray.
China had boasted about 2,600 ECMO machines by the end of last year, data from the National Health Commission showed.